Contrast Media to Pregnant Patients
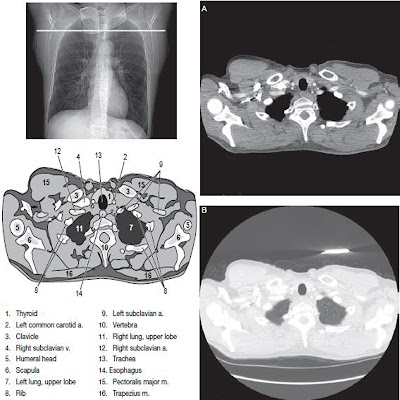
As a result of concerns about exposing the fetus to ionizing radiation, CT examinations are seldom done during pregnancy. But in most rare cases, such examinations may be vital for the mother’s health. Studies of iodinated contrast agents in pregnancy have been limited, and effects on the human embryo or fetus are unknown. Laboratory and animal test of High Osmolality Contrast Media and Low Osmolality Contrast Media, respectively, found no mutagenic or teratogenic effects. Iodinated contrast media have been shown to cross the human placenta and enter the fetus. The SCR committee on Drugs and Contrast Media has reviewed this issue extensively and has prepared a summary of information and recommendations.
 |
Administration of Contrast Media to Pregnant or Potentially Pregnant Patients from American College of Radiology Manual on Contrast Media:
Iodinated Xray Contrast Media (Ionic and Nonionic)
Diagnostic iodinated contrast agent may have been shown to cross the human placenta and enter the fetus when given in usual clinical doses. No Adequate and well controlled teratogenic studies of the effects of these agents in pregnant women have been performed.
In conjunction with the existing American Contrast Media Policy for the use of ionizing radiation in pregnant women, we recommend that all imaging facilities should have polices and procedure to reasonable attempt to identify pregnant patients prior to the performance of any diagnostic examination involving ionizing radiation to determine the medical necessity for the administration of iodinated contrast media. If a patient is known to be pregnant, both the potential radiation risk and the potential added risks of contrast media should be considered before proceeding with the study (Res. 24 1995, ACR Policy)
Although it is not possible to conclude that contrast agents present a definite risk to the fetus, there is insufficient evidence to conclude that they pose no risk. Consequently, the committee recommends the following:
A. The radiologist should confer with the referring physician and document in the radiology report or the patient’s medical records the following:
- That the information requested and the necessity for contrast material administration cannot be acquired via other means. Like ultrasonography.
- That the information needed affects the care of the patient and fetus during the pregnancy.
- The referring physician is of the opinion that is not prudent to wait to obtain this information until after the patient is no longer pregnant.
B. It is recommended that pregnant patient undergoing a diagnostic imaging examination with ionizing radiation and iodinated contrast material provide informed consent to document that they understand the risk benefits of the procedure to be performed and the alternative diagnostic options available to them (if any), and that they wish to proceed.
A very small percentage of the iodinated contrast agent given to a mother will be excreted into breast milk and absorbed by the infant. Therefore, it is believed to be safe for the mother and infant to continue breast feeding after receiving a contrast agent.
Administration of Contrast Media to Breast-feeding Mothers from the American College of Radiology on Contrast Media Manual
Iodinated Xray Contrast Media (Ionic and Nonionic)
The plasma half-life of intravenously administered iodinated contrast medium is approximately 2 hours, with nearly 100% of the agent cleared from the bloodstream within 24 hours. Because of its low lipid solubility, less than 1% of the administered maternal dose of iodinated contrast medium is excreted into the breast milk in the first 24 hrs. Because less than 1% of the contrast medium ingested by the infant is absorbed from its gastrointestinal tract, the expected dose absorbed by the infant from the breast milk is less than 0.01% of the intravascular dose given to the mother. This amount of contrast medium represents less than 1% of the recommended dose for an infant undergoing an imaging study, which is 2mL/kg. The potential risk to the infant include direct toxicity and allergic sensitization or reaction, which are theoretical concerns but have not been reported.
Recommendation
Mother who are breast feeding should be given the opportunity to make an informed decision as to whether to continue or temporarily abstain from breast feeding after receiving intravascularly administered iodinated contrast med
ia. Because of the very small percentage of iodinated contrast medium that is excreted into the breast milk absorbed by the infant’s gut, we believe that the available data suggest that it is safe for the mother and infant to continue breast feeding after receiving such an agent. If the mother remains concerned about any potential ill effects to the infants, she may abstain from breast feeding for 24 hours with active expression and discarding of breast milk from both breast during that period. In anticipation of this, she may wish to use a breast pump to obtain milk before the contrast study to feed the infant during the 24 hour period following the examination.